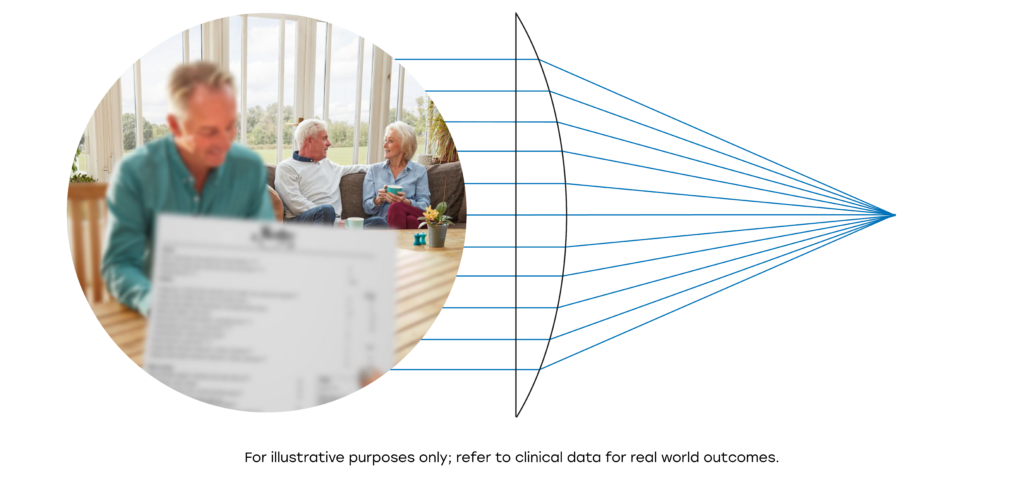
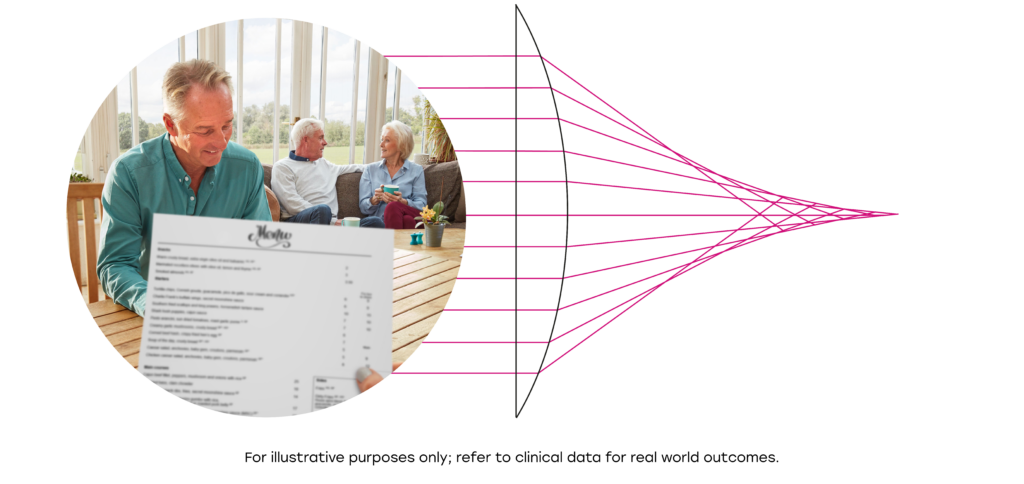
RayOne EMV is a truly non-diffractive IOL which does not use light splitting technology like many IOLs which increase depth of focus, resulting in low levels of dysphotopsia, similar to standard monofocal lenses.1
RayOne EMV is the only patented aspheric IOL that induces controlled positive spherical aberration.
Compared to a lens with zero spherical aberration, the carefully controlled positive spherical aberration induced by RayOne EMV spreads light along the visual axis.
Centre region
Induced positive spherical aberration


Blended edge region
Reduced longitudinal spherical aberration designed to maintain visual acuity and constrast sensitivity under mesopic conditions.

In a robust FDA IDE trial:11
Patients implanted with RayOne EMV Toric had a clinically significant reduction in residual cylinder, meeting the FDA’s recommendation of 0.4 D.
RayOne EMV Toric provided consistently precise astigmatism correction with >85% of patients within ±0.5 D from Month 1 onwards.

Peer2Peer | “My Next Ophthalmologist Needs No Introduction” with Dr Stonecipher (Ep2: Prof Barrett)
Peer2Peer | RayOne EMV US Billing Opportunities
Indication: The RayOne EMV Toric (Model RAO210T) consists of the EMV Toric IOL (210T) preloaded within the RayOne injection system (RAO). The EMV Toric IOL is intended for primary implantation in the capsular bag of the eye for the visual correction of aphakia, in adult patients in whom a cataractous lens has been removed by phacoemulsification and providing reduction of residual refractive astigmatism in adult patients with greater than or equal to 1.00 diopter (D) of corneal astigmatism,compared to a monofocal IOL. The RayOne injection system is used to fold and assist in inserting the IOL into the eye.
RayOne EMV Toric FDA Trial Safety Data | Postoperative Findings
Table A provides a detailed comparison of observed adverse events versus the ISO 11979-7 safety grid. The results of adverse events analyses based on the consensus definitions as set forth by American Academy of Ophthalmology’s Task Force (Masket et al. Ophthalmology 2017) are shown below in Table B. All other ocular adverse events are listed in Table C. Unless otherwise specified, all percentages are based on the Safety Set or subjects implanted with the Toric IOL (N=117) and Monofocal IOL (N=116).
The safety and effectiveness of RayOne EMV Toric (RAO210T) has been demonstrated in an FDA IDE trial.
CAUTION: United States Federal Law restricts this device to sale and distribution by or on the order of a physician and its use is restricted to a properly licensed physician.
Published by Rayner.